


| 产品名称 | Seraseq® ctDNA Reference Material v2 WT(SeraCare) |
|---|---|
| 目录号 | 0710-0208 |
| 别名 | N/A |
| 外观 | N/A |
| 分子量 | N/A |
| CAS | N/A |
| 溶解度 | N/A |
| 存储条件 | N/A |
| 保存时间 | N/A |
| 备注1 | N/A |
| 备注2 | N/A |
| 目录号 | 规格 | 价格 | 库存状态 | |
| 0710-0208 | 1 x 5 mL | 咨询客服 | 咨询客服 |
品名:Seraseq® ctDNA Reference Material v2 WT
货号:0710-0208
品牌:SeraCare
Product Specifications
# of variants:40
Allele Frequency :WT (0%)
Fragment Size:~170bp average
Concentration:25 ng/mL
Volume:5 mL
Total DNA:125 ng (single vial)
Matrix:SeraCon™ Matribase (human plasma-like)
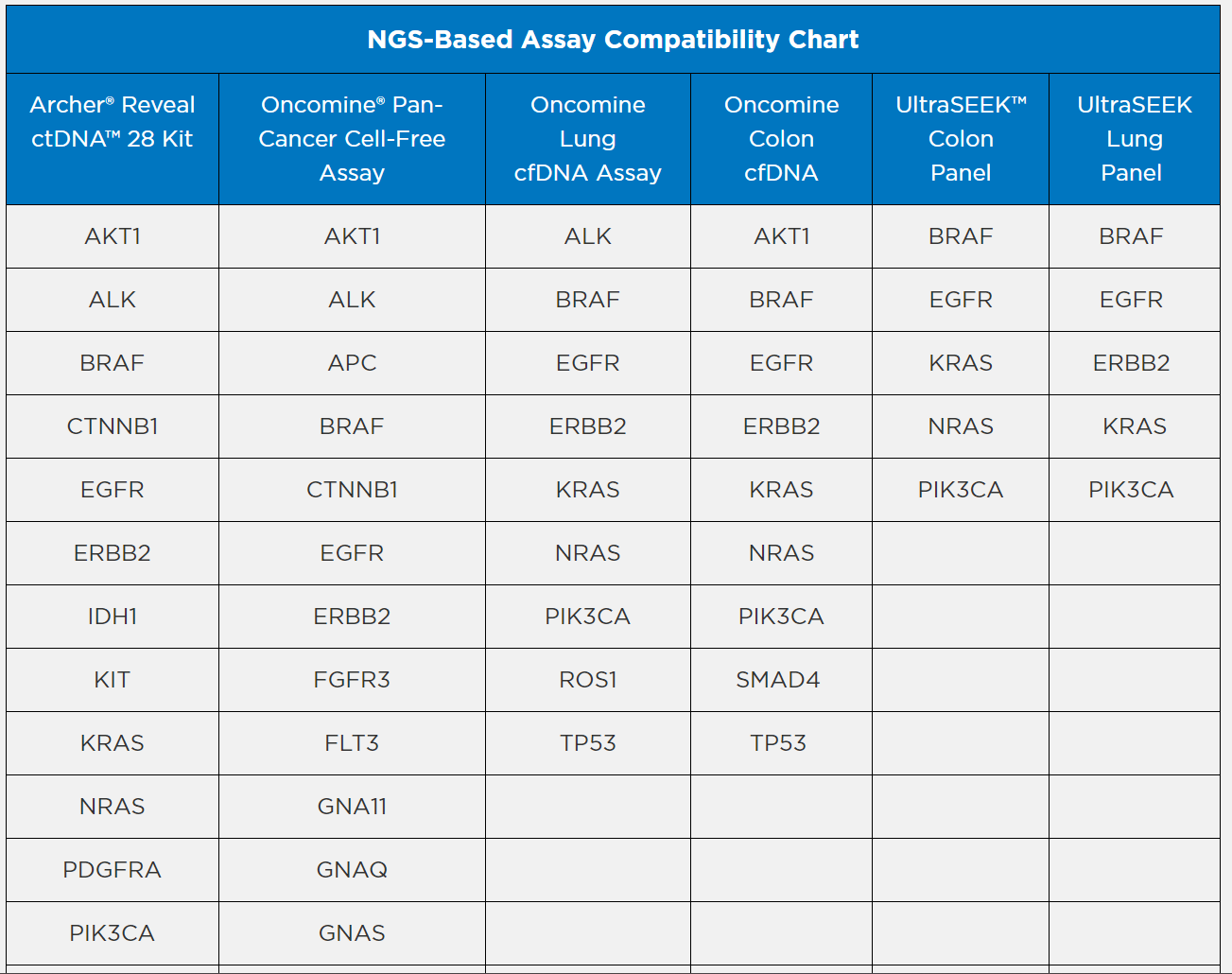
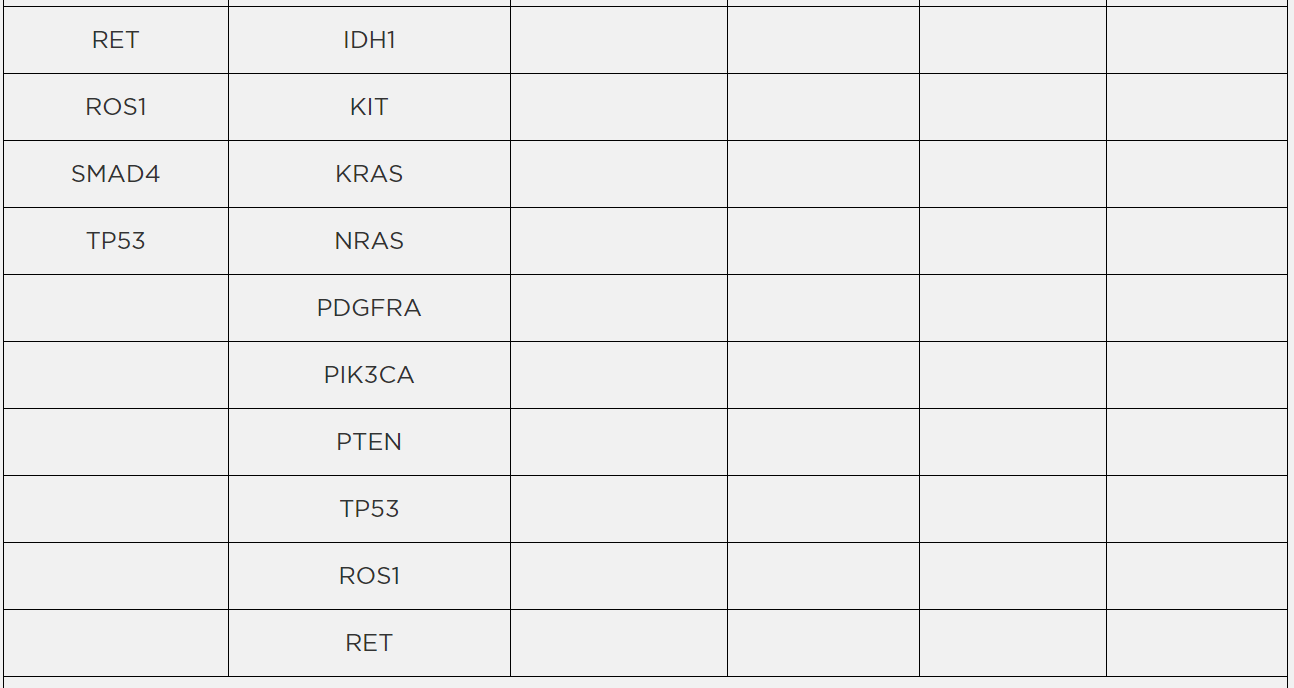
Archer® is a registered trademark, and Reveal ctDNA™ is a trademark of ArcherDx, Inc.
Oncomine® is a registered trademark of Thermo Fisher Scientific.
UltraSEEK™ is a trademark of Agena Bioscience, Inc.
Details
To overcome the lack of patient-like reference materials for liquid biopsy, including drawbacks of existing
methodologies such as sonication, SeraCare has developed a breakthrough technology that produces the
most patient-like materials compared to native ctDNA. The Seraseq ctDNA Mutation Mix v2 is a highly
multiplexed product for NGS-based ctDNA assays targeting cancer-relevant somatic mutations.
This specific ctDNA Reference Material v2 product is the wild-type with an allele frequency of 0%.
Size distribution, library yield and complexity consistent with native ctDNA
Single sample with 40 clinically-relevant mutations across 28 genes, all at the same specified allele frequency
Offered in a full-process plasma-like material capable of assessing the entire workflow from extraction
through analysis
Mutation targets precisely quantitated with digital PCR
Single well-characterized GM24385 human genomic DNA as background wild-type material
Manufactured in GMP-compliant and ISO 13485-certified facilities

维百奥生物代理SeraCare对照及参比物质。
维百奥(北京)生物科技有限公司,自2018年与SeraCare(现隶属于LGC Clinical Diagnostics)合作以来,长期为中国客户
提供包括SeraSeq系列参比物质,领域包括肿瘤、生殖健康和遗传病。SeraCare研发和生产一系列临床参比物质,可以用于任何
研发阶段,以确保临床基因组学检测结果的准确性。
SeraCare参比物质涵盖的范围:
1)Next Generation Sequencing
2)Sanger Sequencing
3)Real-time PCR and digital PCR (dPCR)
4)Microarray
主要特点
1)Ready-to-use reference materials covering clinically-relevant variants and all variant types - SNVs, INDELS, CNVs,
and RNA fusions
2)Highly multiplexed - provide significantly more data per NGS run, saving sequencing costs
3)Available in multiple formats to suite different workflow needs - purified DNA & RNA, ctDNA, encapsulated ctDNA
in plasma, and FFPE
4)Manufactured in cGMP-compliant, ISO 13485-certified facilities
5)Stringent product release testing - all variants qualified by dPCR for allele frequencies or copy numbers