


| 产品名称 | Seraseq® ctDNA Complete™ Mutation Mix AF0.1% (SeraCare) |
|---|---|
| 目录号 | 0710-0532 |
| 别名 | N/A |
| 外观 | N/A |
| 分子量 | N/A |
| CAS | N/A |
| 溶解度 | N/A |
| 存储条件 | N/A |
| 保存时间 | N/A |
| 备注1 | N/A |
| 备注2 | N/A |
| 目录号 | 规格 | 价格 | 库存状态 | |
| 0710-0532 | 1 x 25 µl | 咨询客服 | 咨询客服 |
品名:Seraseq® ctDNA Complete™ Mutation Mix AF0.1%
货号:0710-0532
品牌:SeraCare
Product Specifications
# of Variants 25
Allele Frequency 0.1%
Fragment Size ~170bp
Concentration 10ng/µL
Fill Size 25 µL
Total DNA 250 ng
Buffer Tris/EDTA (TE) NGS-Based Assay
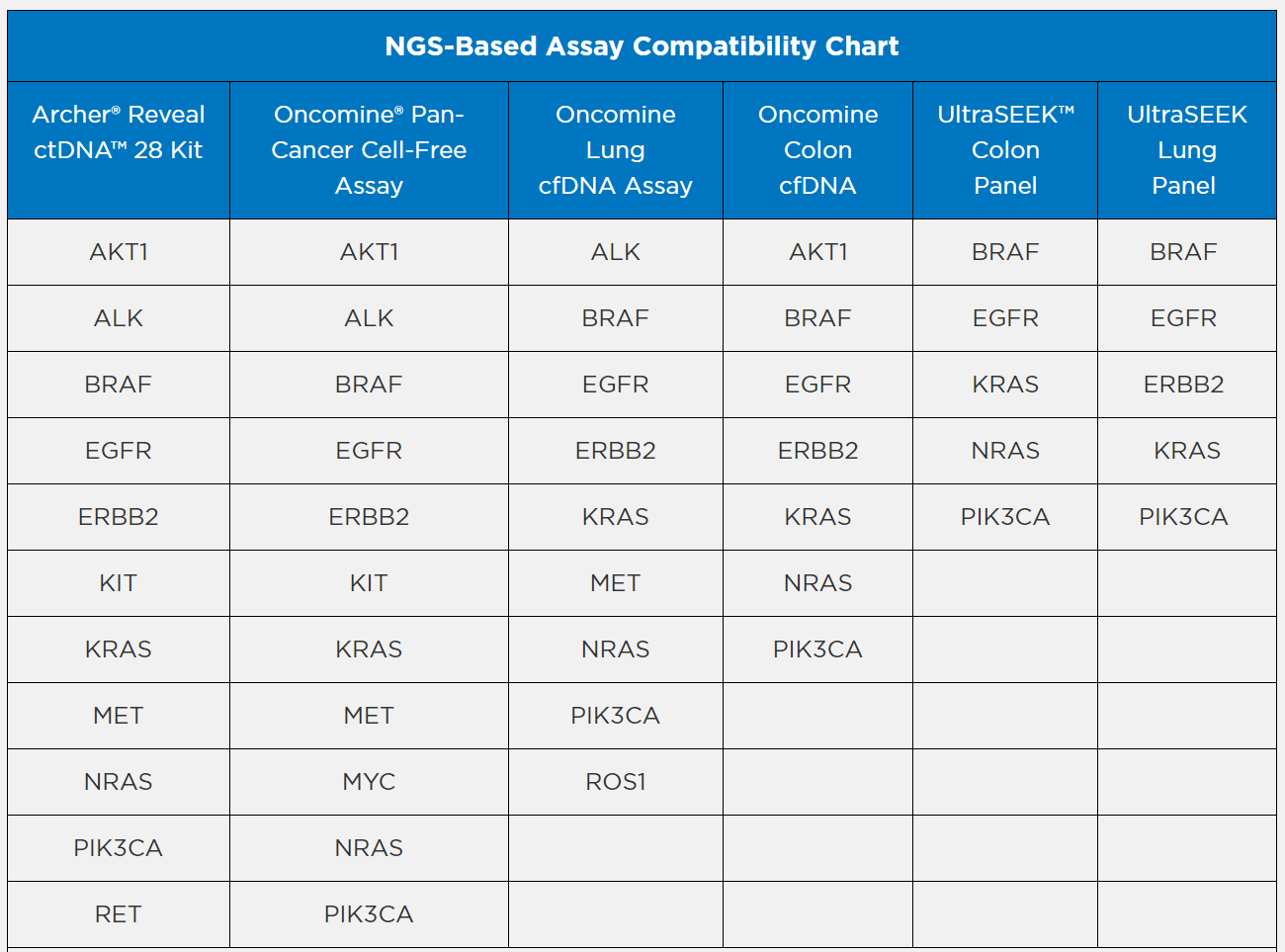
Archer® is a registered trademark, and Reveal ctDNA™ is a trademark of ArcherDx, Inc.
Oncomine® is a registered trademark of Thermo Fisher Scientific.
UltraSEEK™ is a trademark of Agena Bioscience, Inc.
Details
To further accelerate the validation and clinical application of liquid biopsy-based ccfDNA-targeted NGS assays,
SeraCare has developed the Seraseq ctDNA Complete that expands coverage of all relevant variant types including
SNVs, INDELs, CNVs, and gene fusions in a single highly-multiplexed reference sample. These new products are
offered in either a purified ctDNA format or a plasma-like matrix format, all precisely quantitated by digital PCR
against a single well-characterized genomic background (GM24385), and orthogonally validated with NGS
technology.
This specific ctDNA Complete product has an allele frequency of 0.1%.
25 unique multiplexed variants in 16 genes, covering 12 SNVs, 7 INDELs, 3 CNVs, and 3 SVs, in purified and
plasma-like matrix formats.
6 different allele frequencies available: wild-type, 0.1%, 0.5%, 1%, 2.5%, and 5%
Allows end-to-end evaluation of assay performance across the entire workflow, including pre-analytic extraction
steps
Variants quantitated with digital PCR and orthogonally validated by NGS
Blends with well-characterized GM24385 human genomic DNA as background wild-type material
Manufactured within cGMP-compliant and ISO 13485-certified facilities.

维百奥生物代理SeraCare对照及参比物质。
维百奥(北京)生物科技有限公司,自2018年与SeraCare(现隶属于LGC Clinical Diagnostics)合作以来,长期为中国客户
提供包括SeraSeq系列参比物质,领域包括肿瘤、生殖健康和遗传病。SeraCare研发和生产一系列临床参比物质,可以用于任何
研发阶段,以确保临床基因组学检测结果的准确性。
SeraCare参比物质涵盖的范围:
1)Next Generation Sequencing
2)Sanger Sequencing
3)Real-time PCR and digital PCR (dPCR)
4)Microarray
主要特点
1)Ready-to-use reference materials covering clinically-relevant variants and all variant types - SNVs, INDELS, CNVs,
and RNA fusions
2)Highly multiplexed - provide significantly more data per NGS run, saving sequencing costs
3)Available in multiple formats to suite different workflow needs - purified DNA & RNA, ctDNA, encapsulated ctDNA
in plasma, and FFPE
4)Manufactured in cGMP-compliant, ISO 13485-certified facilities
5)Stringent product release testing - all variants qualified by dPCR for allele frequencies or copy numbers