


| 产品名称 | Seraseq® Inherited Cancer DNA Mix v1 (SeraCare) |
|---|---|
| 目录号 | 0730-0003 |
| 别名 | N/A |
| 外观 | N/A |
| 分子量 | N/A |
| CAS | N/A |
| 溶解度 | N/A |
| 存储条件 | N/A |
| 保存时间 | N/A |
| 备注1 | N/A |
| 备注2 | N/A |
| 目录号 | 规格 | 价格 | 库存状态 | |
| 0730-0003 | 1 x 200 µL | 咨询客服 | 咨询客服 |
品名:Seraseq® Inherited Cancer DNA Mix v1
货号:0730-0003
品牌:SeraCare
SPECIFICATIONS
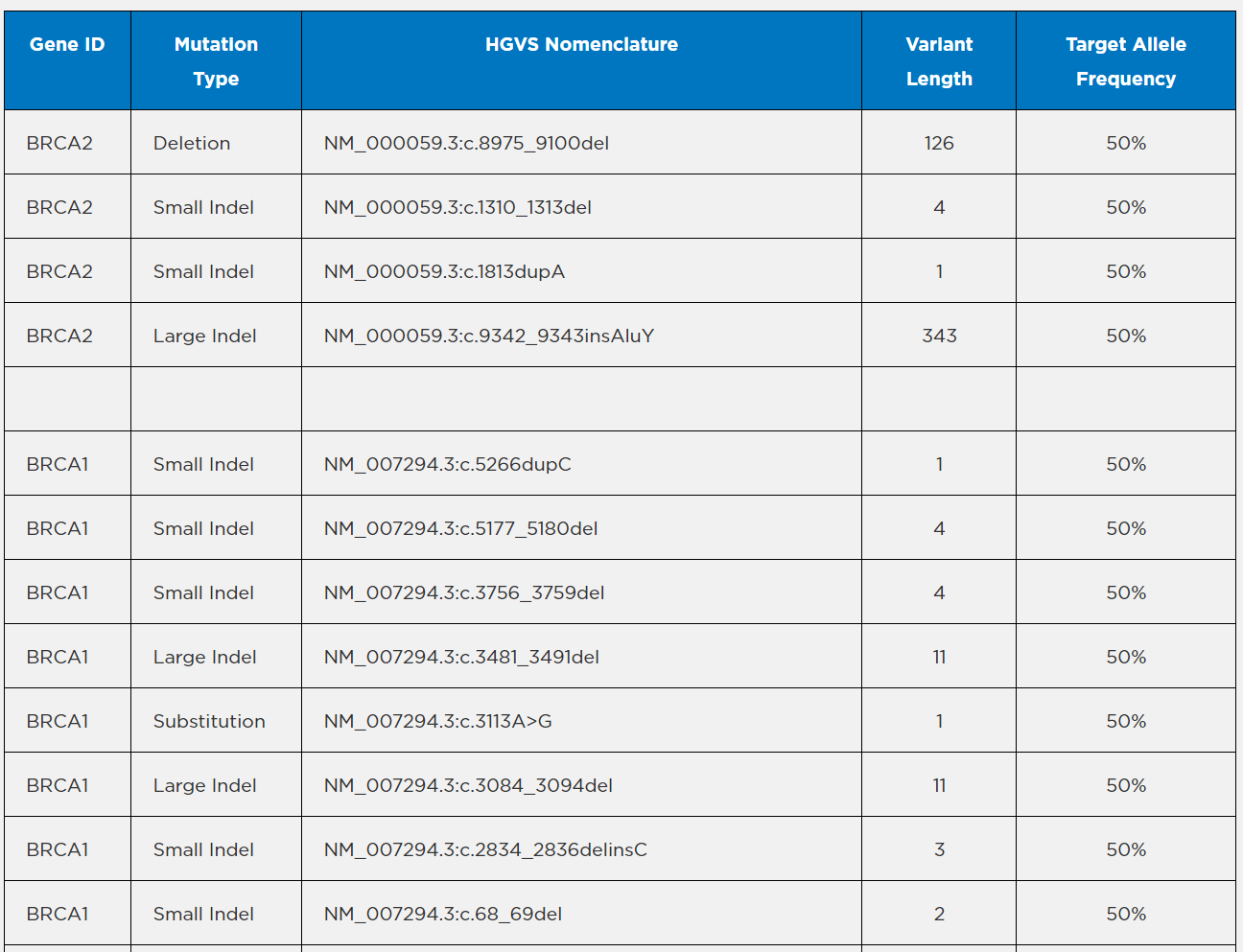
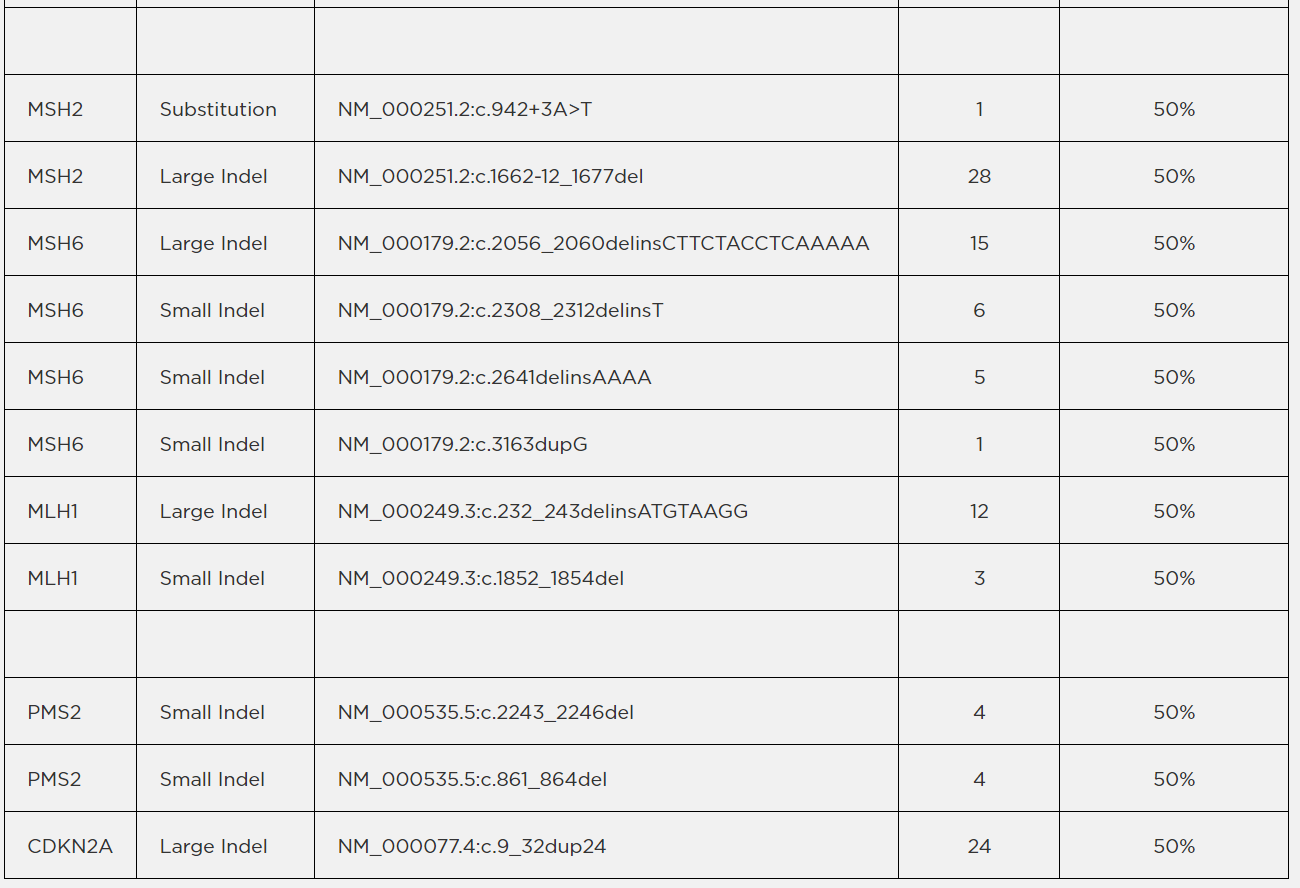
Note: Above list does not include variants present in the GM24385 background. Certain assays may detect the
presence of a PMS2 variant (NM_000535.5:c.2444C>G) which was used for internal development purposes only.
Details
Targeted Next-Generation Sequencing (NGS) panels are increasingly being used to discover causative variants
for inherited cancer, such as Hereditary Breast and Ovarian Cancer. As such panels continue to expand, there
is a growing demand for multiplexed reference materials that cover a broad range of pathogenic variants to
expedite test development and validation. The traditional practice of using reference materials from public
biobanks or remnant patient samples with single pathogenic variants can be extremely tedious, inefficient,
and expensive.
Seraseq Inherited Cancer DNA Mix v1 addresses the lack of multiplexed reference materials for targeted NGS
assays with an expert-designed product1 and published methodology2 focused on seven genes associated
with inherited cancer including BRCA1 and BRCA2.
This unique product combines over 20 variants3 in a well-characterized genomic background that can be used
for assay development and analytical validation.
Expedite assay development and validation with ready source of over 20 inherited cancer-specific variants Save
$1000s in sequencing and validation costs with a highly multiplexed configuration Assess common, rare as well
as technically challenging variants Well-characterized GM24385 human genomic DNA as background wild-type
material Manufactured in GMP-compliant and ISO 13485-certified facilities Customizable to cover desired
variants with the VariantFlex Custom Platform For research use only.
Not for use in diagnostic procedures.
1. Developed in collaboration with Invitae Corp (Steve Lincoln, Rebecca Truty, et al.)
2. Kudalkar EM, Almontarishi NA, Huang C, Anekella B, Bowser M, Hynes E, Garlick R, Funke BH. Multiplexed
Reference Materials as Controls for Diagnostic Next-Generation Sequencing: A Pilot Investigating Applications
for Hypertrophic Cardiomyopathy. J Mol Diagn. 2016 Sep 15. pii: S1525-1578(16)30142-8. doi:10.1016/j.jmoldx.
2016.07.005
3. See Specifications tab for full list of variants.

维百奥生物代理SeraCare对照及参比物质。
维百奥(北京)生物科技有限公司,自2018年与SeraCare(现隶属于LGC Clinical Diagnostics)合作以来,长期为中国客户
提供包括SeraSeq系列参比物质,领域包括肿瘤、生殖健康和遗传病。SeraCare研发和生产一系列临床参比物质,可以用于任何
研发阶段,以确保临床基因组学检测结果的准确性。
SeraCare参比物质涵盖的范围:
1)Next Generation Sequencing
2)Sanger Sequencing
3)Real-time PCR and digital PCR (dPCR)
4)Microarray
主要特点
1)Ready-to-use reference materials covering clinically-relevant variants and all variant types - SNVs, INDELS, CNVs,
and RNA fusions
2)Highly multiplexed - provide significantly more data per NGS run, saving sequencing costs
3)Available in multiple formats to suite different workflow needs - purified DNA & RNA, ctDNA, encapsulated ctDNA
in plasma, and FFPE
4)Manufactured in cGMP-compliant, ISO 13485-certified facilities
5)Stringent product release testing - all variants qualified by dPCR for allele frequencies or copy numbers