


| 产品名称 | Seraseq® Cardiomyopathy Reference Material (SeraCare) |
|---|---|
| 目录号 | 0740-0021 |
| 别名 | N/A |
| 外观 | N/A |
| 分子量 | N/A |
| CAS | N/A |
| 溶解度 | N/A |
| 存储条件 | N/A |
| 保存时间 | N/A |
| 备注1 | N/A |
| 备注2 | N/A |
| 目录号 | 规格 | 价格 | 库存状态 | |
| 0740-0021 | 1 x 200 µL | 咨询客服 | 咨询客服 |
品名:Seraseq® Cardiomyopathy Reference Material
货号:0740-0021
品牌:SeraCare
SPECIFICATIONS
Mutations included in the Seraseq Cardiomyopathy Reference Material
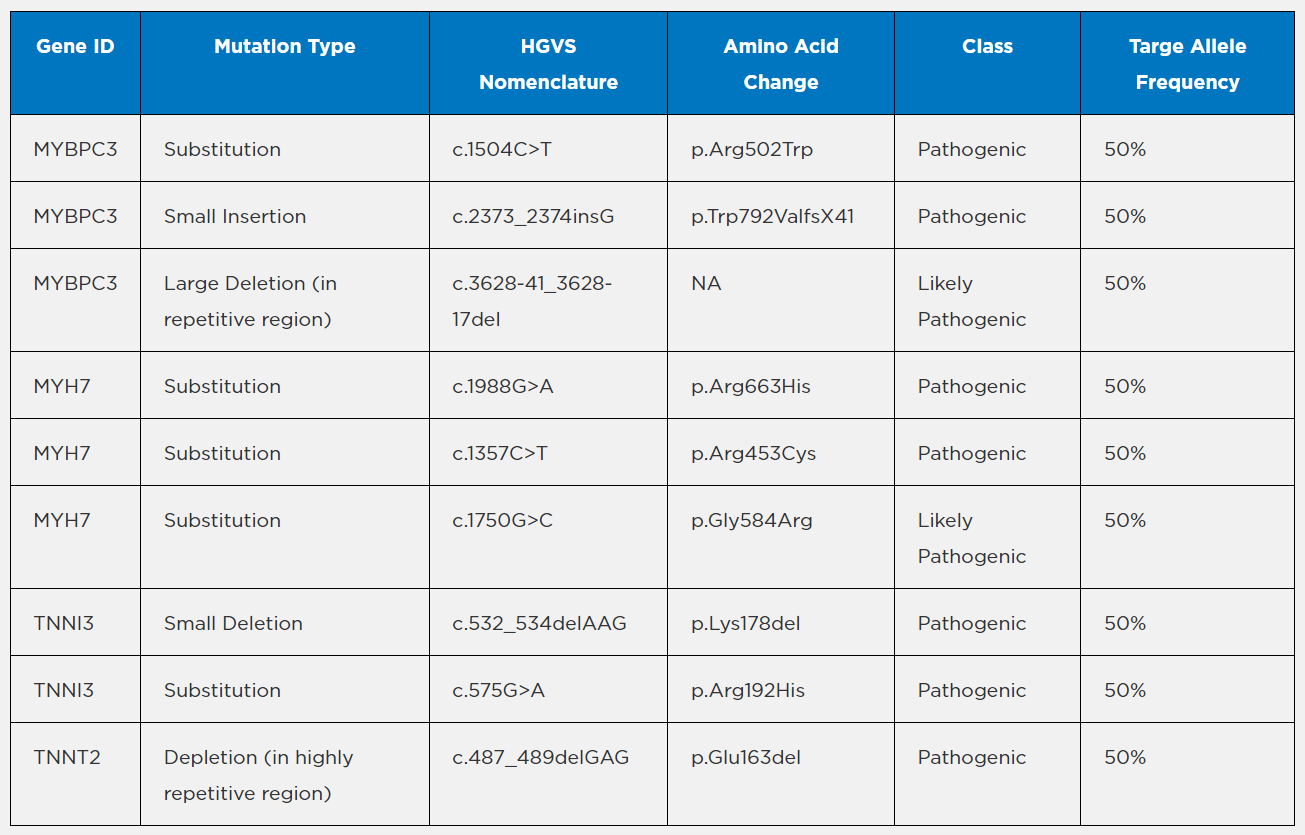
Details
Seraseq Cardiomyopathy Reference Material addresses the lack of multiplexed, patient-like reference materials
with an expert-designed product1 for targeted NGS assays focused on hypertrophic cardiomyopathy (HCM).
This unique product combines ten actionable HCM mutations in a well-characterized genomic background
and can be used for assay development, validation, and to support lab QC standardization.
Key features include:
Ten variants considered pathogenic or likely pathogenic for HCM in a single sample
Mutation targets precisely quantitated with digital PCR
Well-characterized GM24385 human genomic DNA as background wild-type material
Manufactured in cGMP compliant and ISO 13485-certified facilities
References:
1. Emily M Kudalkar, Naif AM Almontarishi, Catherine Huang, Bharathi Anekella, Mark Bowser, Elizabeth Hynes,
Russell Garlick, Birgit H. Funke. Multiplexed reference materials as controls for diagnostic next generation
sequencing – a pilot investigating applications for hypertrophic cardiomyopathy, The Journal of Molecular
Diagnostics.
For research use only. Not for use in diagnostic procedures.

维百奥生物代理SeraCare对照及参比物质。
维百奥(北京)生物科技有限公司,自2018年与SeraCare(现隶属于LGC Clinical Diagnostics)合作以来,长期为中国客户
提供包括SeraSeq系列参比物质,领域包括肿瘤、生殖健康和遗传病。SeraCare研发和生产一系列临床参比物质,可以用于任何
研发阶段,以确保临床基因组学检测结果的准确性。
SeraCare参比物质涵盖的范围:
1)Next Generation Sequencing
2)Sanger Sequencing
3)Real-time PCR and digital PCR (dPCR)
4)Microarray
主要特点
1)Ready-to-use reference materials covering clinically-relevant variants and all variant types - SNVs, INDELS, CNVs,
and RNA fusions
2)Highly multiplexed - provide significantly more data per NGS run, saving sequencing costs
3)Available in multiple formats to suite different workflow needs - purified DNA & RNA, ctDNA, encapsulated ctDNA
in plasma, and FFPE
4)Manufactured in cGMP-compliant, ISO 13485-certified facilities
5)Stringent product release testing - all variants qualified by dPCR for allele frequencies or copy numbers